An epoch-making technology involving “asbestos visualization”
with fluorescent-labeled asbestos-binding protein/peptide
Professor Kuroda’s laboratory conducts research in three fields: biomaterial/biosystem interfaces, phosphorus and biosystems, and silicon and biosystems. These fields are of interest in biosensor※ development, which can contribute in the arenas of environmental biotechnology and health. In this interview, we focus on his research in the field of biomaterial/biosystem interfaces.
- ※A measurement technology based on the excellent molecular recognition capacity of living organisms and biomolecules.

Professor Kuroda is renowned for the development of the biofluorescence method, which can detect asbestos under a fluorescent microscope. The issue of asbestos has attracted widespread attention since 2005. Asbestos is a fibrous mineral that has long been used as a construction material. The health hazards to people working in the production of asbestos and on construction using asbestos-containing materials have been widely publicized. Today, many developed countries have completely prohibited the use of asbestos. However, some old buildings built using asbestos-containing materials are still standing; when they are torn down, there is the risk of releasing asbestos, which needs to be monitored on-site. Thus, asbestos remains an urgent issue.
The conventional asbestos detection method, which identifies the particle by element analysis using an electron microscope and X-ray, requires a high skill level, and the process is complicated. Thus, the development of an easier and more rapid detection method is required. Professor Kuroda’s idea was to look for a protein or peptide that specifically binds asbestos in order to detect it, and his research group was focused in this direction. According to the professor, “An easy and simple solution is to create an antibody that monitors asbestos. However, it is difficult to create such antibodies because asbestos is an inorganic material. Therefore, we need to look for a protein/peptide that specifically binds to asbestos in different ways. Since we have already found a protein that binds to a silicon semiconductor, it might not be difficult to find a protein that binds to asbestos, because asbestos is a silicon-containing material.”
Eventually, Professor Kuroda’s research group found an asbestos-binding protein. Then, through genetic manipulation, they fused the asbestos-binding protein to a fluorescent protein. This fluorescent-labeled protein enabled them to rapidly detect asbestos under a fluorescent microscope, in what they named the biofluorescence method.
 Asbestos visualization using biotechnology
Asbestos visualization using biotechnology
 A portable iPad fluorescent microscope
A portable iPad fluorescent microscope
In 2010, the Kuroda research group developed an Asbestos Detection Kit. In 2016, they succeeded in developing a portable iPad fluorescent microscope, in which non-skilled operators can receive technical support for a real-time assessment from the inspection room. The biofluorescence method was officially certified by the Ministry of the Environment (Japan) in 2017. This kit is the first in the world to use proteins or peptides to capture plane surfaces of inorganic materials for detection of a particle of interest.
 The commendation for Science and Technology by the Minister of Education, Culture, Sports, Science and Technology, 2012 (left), and Industry, Government, and Academia Cooperation Merits by the Minister of the Environment, 2017 (right)
The commendation for Science and Technology by the Minister of Education, Culture, Sports, Science and Technology, 2012 (left), and Industry, Government, and Academia Cooperation Merits by the Minister of the Environment, 2017 (right)
Collecting/refining exosomes using an exosome-binding peptide
for medical applications
Professor Kuroda is also studying exosomes with Lecturer Ishida. An exosome is an extracellular vesicle with a diameter of 30–120 nm, with a lipid bilayer membrane, and is secreted from cells. Exosomes can deliver molecules such as microRNAs (miRNA). With the protective outer layer of the exosome surrounding the miRNAs, cellular information is preserved intact. The state of certain diseases can be analyzed based on the miRNAs contained in blood exosomes. As such, researchers have started using exosomes as a new biomarker for detecting cancer. In addition, these scientists are also using exosomes as a treatment because exosomes of stem cells have anti-inflammatory potential. However, it is challenging to collect (refine) exosomes at a high throughput and/or on a large scale. A method to efficiently prepare exosomes with minimal damage is required; this prompted Professor Kuroda’s research group to initiate a project to identify a solution to this issue.
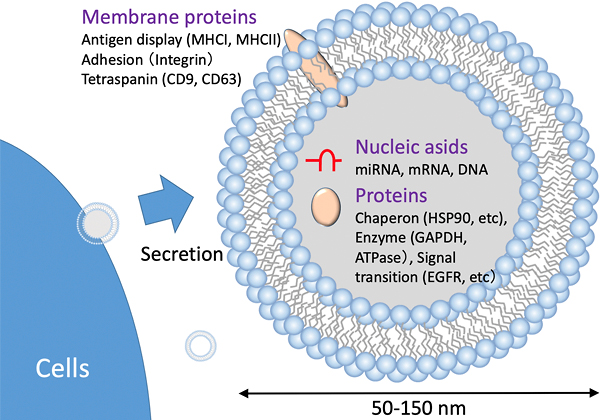 Schematic of an exosome
Schematic of an exosome
They use a peptide that binds to lipid bilayers, which are organic interfaces.
As the professor explained, “We finally found that a peptide that contains eight lysine residues, called EX peptide, is the most suitable because of its moderate affinity with the exosome bilayers.”
It is possible to collect and refine exosomes from blood serum with very little damage using magnetic beads immobilized with the EX peptide. Kuroda et al. have developed an exosome refining kit and an automatic exosome-refining system that are already in the market. They have also created columns for large-scale exosome refinement, which will contribute to exosome medical applications.
 Schematic of refining exosomes using EX peptide
Schematic of refining exosomes using EX peptide
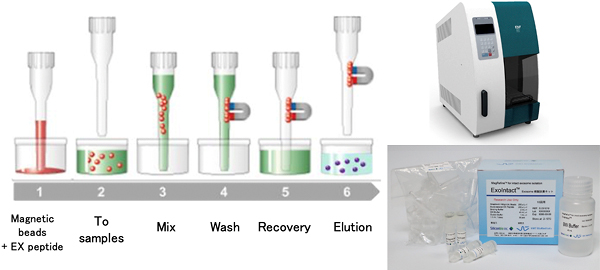 Automatic exosome refining system
Automatic exosome refining system
The common theme in Professor Kuroda’s research is
to capture inorganic/organic surfaces
The key to this research is to use proteins/peptides that capture plane surfaces. However, in principle, such substances do not exist in cells. There seemed to be no proteins/peptides that bind asbestos in nature. Professor Kuroda explained this as follows:
“Specific binding between proteins occurring in a living body has evolved based on the recognition of their uneven and non-flat structures.” On the other hand, “artificial peptides offer great possibilities.”

“Artificial peptides have great possibilities.”
He continued, “Supposing that there is a series of peptides composed of 100 amino acids (20 types of amino acid), the entire variety of these peptides is 20 to the 100th power, which is far more than the number of atoms in the universe, which is said to be 10 to the 90th power.” Even if all the substances in the universe were peptides, they would not cover all possible types. There are a tremendous number of types of peptides, and we know only a fragment of them. Therefore, randomly combining unknown types, which do not exist in nature, has great potential when we look for peptides that can capture plane surfaces.”
According to the professor, little research on peptides that recognize plane surfaces is done anywhere.
He said, “Asbestos has a plane of inorganic crystal, and an exosome is a plane of lipid bilayers. We are looking for a peptide that captures the plane surfaces of various inorganic substances and semiconductors. The basis of this research is to find proteins/peptides that bind plane surfaces and to use them for practical applications.”
“It is a great joy to create things that do not exist in the world and contribute to people’s lives,” said Professor Kuroda. “Being asked ‘How did you think of such an idea?’ is the most exciting moment for me.” Finally, he added, “Diversity is important for an academic university. I hope that young students will see the research that no one has conducted as a challenge, rather than seeking easy results.”
■YouTube
 Opened on November 29, 2021
Opened on November 29, 2021


