First, she had an interest in such a microscopic
world that is invisible even with microscopes.
Professor Villeneuve’s research area is colloid and surface chemistry. Her major is thermodynamics.
“Two materials in contact with each other always form an interface. When one of the materials is air
or vacuum, the interface is commonly called ‘surface.’ In reality, the terms surface and interface
refer to the same thing,” says the professor.
According to the professor, interfaces are present only between two adjacent layers. They are only a
few nanometers thick. Despite their existence in extremely thin regions comparable to a few
molecules, they are involved in various phenomena in the natural world. She describes the interfaces
as being “in a mysterious domain that is invisible with microscopes.” Professor Villeneuve desires
to elucidate various phenomena taking place at the interfaces and wishes to understand living things
by means of basic research.

The professor became interested in this field when she learned about micelle colloids in a high-school chemistry class. “When, in a class, I was listening to a story about hydrophilic and hydrophobic colloids, I found it interesting that molecules assemble to form a material by virtue of a relatively weak force rather than strong bonds like covalent bonds,” says Professor Villeneuve.
The term micelle refers to an aggregate of surfactant molecules. In water, each micelle forms into a spherical shape, with its hydrophilic groups on the outer side and hydrophobic groups on the interior. A dispersion of particles approximately 1 nm to 1 µm in diameter in water is known as a “colloid.” Milk and muddy water are colloids. At a certain level of or higher concentration in a solution, surfactant molecules form an association colloid, which is known as a micelle. Taking soap as an example, high school students learn that soap forms micelles. Professor Villeneuve’s research is an extension of that idea. According to her, “research on soap in actuality isn’t so simple.”
Soap is produced by mixing fatty acids with an alkali, such as sodium carbonate. Depending on the mixing ratio of the alkali, association colloids are created into substantially different forms. Professor Villeneuve studies the effects of different concentrations and pHs on the forms of association colloids, such as by creating lithium soaps using lithium as an alkali.
The basic unit of these associations is the bilayer. It resembles a soap bubble film. Soap bubble films are “thin films that have hydrophilic groups on the interior and hydrophobic groups on the outer surface with water in between.” According to the professor, association bilayers formed in water are a reverse of the soap bubble film. The bilayer has the same structure as that of cell membranes in living organisms. As such, using surfactants, research is undertaken with the goal of elucidating correlations between the functionalities of membranes and membrane structures in living bodies.
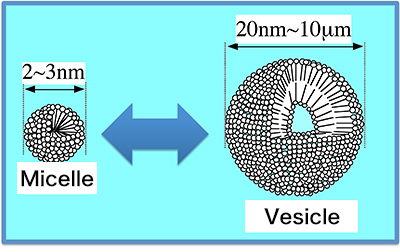 Fig. 1: Vesicle-micelle
transition
Fig. 1: Vesicle-micelle
transition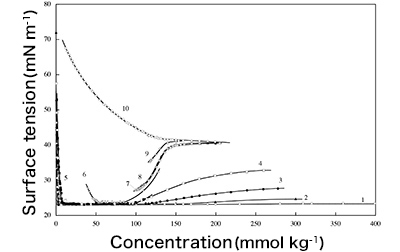 Fig. 2: Research on
vesicle-micelle transition mechanisms through surface tension measurements
Fig. 2: Research on
vesicle-micelle transition mechanisms through surface tension measurements
Colloid and Interface Chemistry Utilized in Rice Bread:
Surface tension works to retain swelling.
The most famous among Professor Villeneuve’s research achievements is “Research on development of a technique for making gluten-free 100% rice-flour bread.” Gluten, a group of proteins contained in wheat flour, is an allergen. In recent years, demand has been growing for gluten-free rice bread. Commercially available rice bread has mostly contained wheat gluten or a thickener as an additive for aiding in the swelling of the bread or used some specific type of manufacturing equipment. In response to the demand, the professor studied how it would be possible to make tasty bread using 100% rice flour in a joint research program with an outside organization. The research successfully resulted in the development of a bread-making technique. The technique utilizes the specific properties of an interface, in which Professor Villeneuve specializes.
“One general stereotype is that it is not possible to bake bread unless you make a dough similar to that of wheat flour bread,” points out the professor. However, according to her, mechanisms underlying bread swelling are different between wheat and rice bread loaves, so the doughs appear differently and baked bread loaves completely differ in structure. In wheat bread, carbon dioxide gas produced through fermentation enters gluten meshes to sustain the dough. In contrast, in rice bread, approximately 5 µm starch particles adhere around carbon dioxide gas produced through fermentation to hold bubbles in the batter.

When considering the mechanisms working for rice bread to rise, Professor Villeneuve looked at scanning electron micrographs of rice bread batter before baking. In the micrographs, she saw a phenomenon known as Pickering foam, which stabilizes bubbles by virtue of adsorption of particles on bubble surfaces. The phenomenon is the stabilization, by starch particles, of boundaries, or interfaces, between fermentation gas and liquid-like batter. The key factor in the adsorption is surface tension. Surface tension works to reduce the area of an interface that has extra free energy. Surfactant molecules present in a liquid and adsorbed onto the interface reduce the free energy and maintain the interface. In rice bread batter, starch particles adsorbed like a surfactant stabilize the batter.
To further investigate the details, the professor’s research team measured the surface tension of water, dispersing rice flour in it. They found that rice flour suitable for bread-making evidently differed from that unsuitable for bread-making in the manner in which surface tension decreased. “Rice is milled either by a wet method, which grinds wet rice, or by a dry method, which grinds dry rice as it is. These types of milled rice flour are chemically identical but differ in physical conditions. This difference leads to differently decreasing surface tension and is conductive to properties either suitable or unsuitable for bread-making.” Based on this finding, the research team used wet-milled rice flour as an ingredient with a low degree of damage to starch. Moreover, they put some thought into fermentation and baking processes. As a result, they successfully made 100% rice-flour bread without using any additional ingredient.
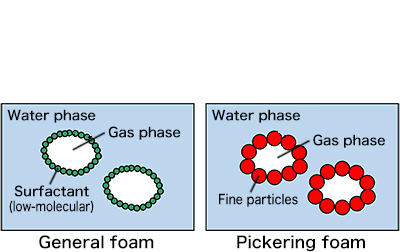 Fig. 3: Structural comparison of Pickering foam and general foam
Swelling mechanisms of gluten-free 100% rice-flour bread
Interfacial stability between water and bubbles
Fig. 3: Structural comparison of Pickering foam and general foam
Swelling mechanisms of gluten-free 100% rice-flour bread
Interfacial stability between water and bubbles
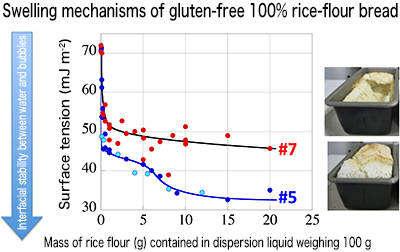 Fig.
4: It is important that rice flour adequately reduces the surface tension of the dispersion
aqueous solution.
Fig.
4: It is important that rice flour adequately reduces the surface tension of the dispersion
aqueous solution.Measurements conducted firsthand are valuable.
I also analyze the dynamic aspects of interfaces.
Professor Villeneuve’s research is characterized by surface tension measurements, as seen in her research on rice bread. What is more, she uses a self-made measurement system. She is confident that “in measuring capacity, the system is more accurate than commercially available systems probably by an order or two of magnitude.” “Some things become visible only through accurate measurements,” says the professor.
Meanwhile, with advances in visualization technology, many researchers in her area of specialization tend to quit using surface tension measurement. Regarding this trend, Professor Villeneuve believes that “although visualization technology appears to facilitate understanding, one can understand interactions between solvent and substances only through accurate measurements of surface tension, which is extra energy, or what is called Gibbs free energy.” For this reason, she devises techniques, such as using microscopes and spectroscopy that provide complementary information to thermodynamics, while adhering to research techniques based on surface tension measurements.
“I have recently begun taking another approach that involves measuring the viscoelasticity of interfaces,” says the professor. She says, “by knowing viscoelasticity that represents dynamic properties in addition to through the conventional practice of collecting thermodynamic data, I would like to look at both static and dynamic aspects of interfaces.”

In the first place, the study of interfaces is a cross-disciplinary research area relating to plural areas of studies. It requires knowledge extending beyond the boundaries of chemistry or physics. Professor Villeneuve encourages students: “When I was a youthful college student, I wondered why it was necessary to study mathematics, such as linear algebra, and physics for the sake of chemistry. However, I truly felt the necessity when I was enrolled in an advanced course. I hope that students who are interested in this area of study will acquire a variety of knowledge from a broad perspective.”
Her attitude as a researcher remains unchanged: “I strive to make new discoveries while adhering to the basics.” She is an experiment lover, spending hours conducting experiments. Even when she obtains experimental results that differ from her hypothesis, she finds herself being happy because she has “gotten a new discovery.”
“In living things and in nature, there are many phenomena relating to interfaces. Such phenomena are sometimes found in unexpected places. For instance, principles of colloid sciences apply to everyday items, such as cosmetics and automobiles, as well as to cell membranes. I am willing to continue exploring this area of study, which will lead to an understanding of living things and nature and be relevant to our lives.”
 Opened May 18, 2020
Opened May 18, 2020


