Clarifying intracellular protein structures from the perspective of fluctuations
Professor Tate is an authority on biophysics, an area of science that explores the fundamental roots of life. In particular, Professor Tate focuses his research on molecules, especially intracellular proteins.
"My interest centers around the understanding of life through molecules. What motivates me to be involved in research activities is the aspiration to describe and understand the phenomena of life in physical and mathematical languages," says Tate.
His research aims at clarifying how the physical characteristics, movements and transformations of molecules are related to the control of the phenomena of life. The primary tool for this quest is NMR (nuclear magnetic resonance), which the professor has studied since his student days.
It is well known that proteins have specific 3D structures, which play a critical role in the functional expression of molecules. After completion of the project to decode the human genome was declared in 2003, it has been revealed through the results of exhaustive genome sequencing that 50% of proteins in mammals were in natural alteration regions (ID regions) that do not retain stable 3D structures. "This means that the history of structural biology over the past 70 years has focused on only one of the sides of protein molecules," says Tate. Attention has subsequently been drawn to structural analysis, and to functional analysis based on structural analysis, regarding the structures and functions of the remaining 50% of proteins (the parts of proteins that do not have fixed forms). Researchers across the world are interested in this, including Professor Tate, who is pursuing the clarification of functional roles that fluctuations in protein structures play, with particular focus on the identification of new functions that ID regions have. It is characteristic that he is promoting proprietary research on new protein structures, based on the development of new structural analysis technology.
"NMR is characteristic in that it is capable of identifying a protein structure in solution. On the other hand, it is not able to handle excessively large molecules. Proteins that are targeted in pharmaceutical development exceed the molecular weight limit of 30 kDa that can be structurally analyzed by NMR. Therefore, the development of a new technology is inevitable at this point. This is why our team promoted the development of an NMR technology targeted at such high molecular weight proteins, for the purpose of observing the molecular transformation that expresses when proteins function, in a quantitative manner and with high sensitivity."
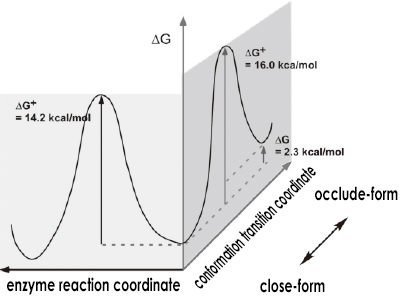 Fluctuation and enzyme reaction control in the two time domains of DHFR activity loop
structure
Fluctuation and enzyme reaction control in the two time domains of DHFR activity loop
structure

 AVANCE 700 MHz Digital NMR System
AVANCE 700 MHz Digital NMR System
Successfully developed an NMR technology for observing
the molecular transformation of high
molecular weight proteins
Professor Tate named the new measurement technology "DIORITE (Determination by Induced ORIentation by Trosy Experiments)." The DIORITE method utilizes "anisotropic spin interaction," which is not used in normal NMR. Professor Tate says that they overcame the molecular weight limit by determining molecular orientation tensor based on the magnetic field orientation-dependent changes of Trosy (Transverse Relaxation Optimazed Spectroscopy) signals that could be observed with high sensitivity even for high molecular weight proteins. (*1)
"Plainly speaking, the parts of proteins that have fixed forms have been clarified through X-ray crystal structure analysis, and an enormous amount of 3D structural information has been accumulated in the protein data bank. On the other hand, in the parts of proteins that do not have fixed forms, i.e., those do not retain stable 3D structures, there are cases where masses are connected in beads. It is extremely important to know how such masses spatially change their directions. Analysis on this point was extremely difficult. The DIORITE method is a measurement technology that utilizes NMR, capable of extremely accurate analysis concerning how the masses connected in beads spatially change their positions, and how their directions are altered when drugs interfere with that process," says Tate.
Verification experiments have proved that this technology exercises adequate analysis accuracy in practice if optimal conditions are properly provided by leveraging other technologies, such as sample adjustment technology for preparing the optimal magnetic field orientation states for various proteins, which was also developed by Tate's research group. Applications have already been filed for domestic and international patents for this innovative DIORITE method.
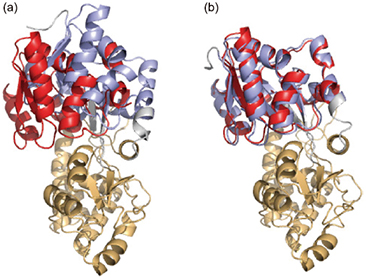 Orientation of the N domain (red) of maltose-binding protein (MBP) as determined by the
DIORITE method
Orientation of the N domain (red) of maltose-binding protein (MBP) as determined by the
DIORITE methodComparison of (a) Apo MBP crystal structure (PDB: 10MP)(blue) and (b) maltose-binding protein (MBP) crystal structure (PDB: 1ANF)(blue)
Retaining unparalleled data and pursuing the visualization
of intranuclear chromatin structure
and
dynamics
"We also have an unparalleled advantage that is absent among our competitors," adds Professor, which refers to the abundant data that they possess as an outcome of research conducted by the Mathematical Research Base for Chromatin Dynamics, which was established at Hiroshima University in a project adopted by the Ministry of Education, Culture, Sports, Science and Technology in 2012.
"We inserted fluorescent labels into 133 gene locuses in Schizosaccharomyces. We have a database that
consists of all several tens of thousands of data pieces concerning the intranuclear movements of
those fluorescent labels. We are confident of these unparalleled data that nobody else in the world
possesses."
By interpreting electronic microscope images utilizing these data, it becomes possible to formulate
a "data-driven model" for chromatin structure based on experimental data. Combining this with an
approach of a mathematical model, Professor Tate says that they can make a significant step toward
the "visualization of intranuclear chromatin structure and dynamics," which is another research
theme that the professor has focused on for many years.
The Mathematical Research Base for Chromatin Dynamics has gathered researchers in experimental sciences specializing in cellular biology and molecular biology, as well as researchers of mathematical sciences who establish mathematical models and promote physical mechanisms based on measurement data. They have undertaken research activities in various fusion areas through close collaboration, and this framework remains functional.
"Research initiatives on a large scale, conducted by a large research group, leads to state-of-the-art research. When we stringently question what problems are of the utmost priority at present, it is absolutely impossible for any single researcher to answer such questions by him/herself, which calls for team research activities that involve a number of researchers."


While Professor Tate's faith in research remains unchanged, his methods and research targets continuously change for these reasons. Tate even considers that the newly developed NMR technology "already belongs to the past," and is moving forward with his main interest in how far he can proceed with electronic microscopy.
This attitude of his is reflected in the composition of his laboratory. At present, half of his
laboratory is engaged in NMR-based research, while the other half handles research by electronic
microscopy. Some of his students are sent to NIH (U.S. National Institutes of Health), Seoul
National University, or other institutions for acquiring sample preparing techniques for electronic
microscopy.
How far will his research continue to advance? In conclusion, we asked Professor Tate to share his
outlook with us.
"Our short-term goal is to achieve the complete visualization of intranuclear chromatin structures. I want to fully understand how chromosomes transform, and how they are related to genetic control and cell differentiation, and how they respond to stimulation from outside, and so on. We would call it a milestone if we could achieve the relevant modeling. I would like to reach that milestone before my retirement. We are using yeast at present. Ultimately, we hope to handle human cells, envisioning future application in medicine. I suppose that successful advancement in this course would necessitate technological innovation in electronic microscopy itself. This is beyond my specialty, and I depend on specialists in that field."
Regarding research concerning intranuclear chromatin structure analysis, which he is promoting in collaboration with a research group at NIH, Professor Tate smiled, commenting, "We have made certain achievements, and cannot wait for their publication."
*1. For more details, see "Molecular Forms of Ultra High Molecular Weight Proteins Observed by NMR" at https://www.jstage.jst.go.jp/article/biophys/51/2/51_2_084/_pdf(in Japanese).
 Opened on June 1, 2020
Opened on June 1, 2020


